Purification of water from organic iron
Purification of water from organic iron. Contamination of groundwater with organic substances is caused by the ingress of decay products of living organisms.
The main signs of the presence of organo - iron compounds in water are such indicators as:
- Increased permanganate oxidizability (more than 5 units).
- Increased color (more than 20). The water has a color from yellowish to light brown.
- Increased turbidity.
- Unpleasant smell (the smell of the swamp).
- With prolonged settling in an open container, the water does not lighten, the sediment does not fall out.
An effective and efficient method of removing organo-iron compounds is coagulation with aluminum polyoxychloride (aquaaurate) or aluminum sulfate. Also, in most cases, water with high color and with a large amount of organic matter has a low PH (hydrogen index). Its normalization, correction in the direction of increase, significantly accelerates coagulation. To do this, in addition to aquaaurate, alkali (caustic soda) is also added to the water.
Chemical reactions between the coagulant and colloidal substance dissolved in water convert these substances into an insoluble precipitate in the form of flakes.
The scheme of water purification by coaculation can be in pressure and non-pressure versions. In the pressure unit, the coagulant is dosed directly to the inlet of the sedimentary filter.
Water purification by pressure coagulation
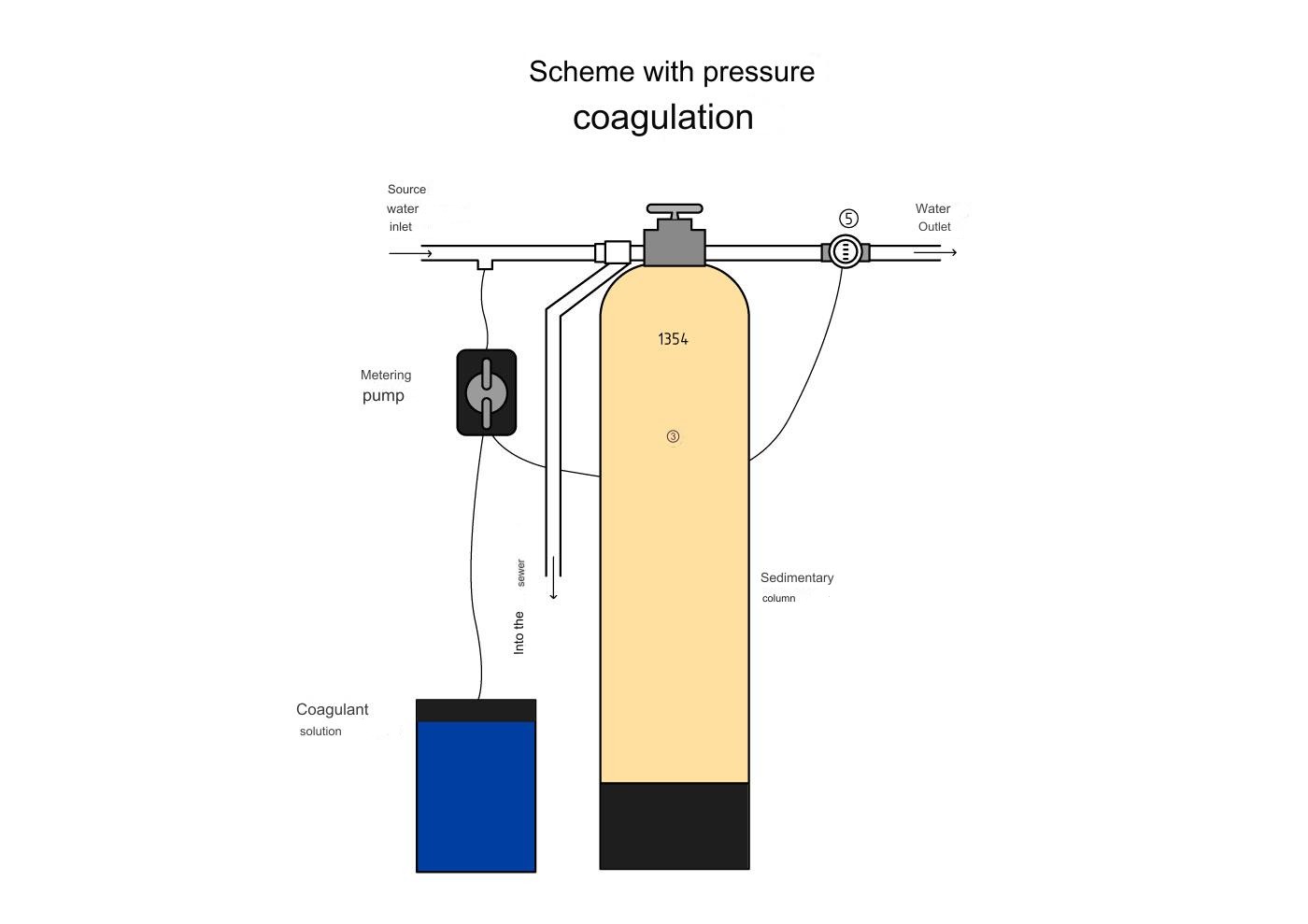
The main disadvantages of the pressure coagulation cleaning scheme are as follows:
- All the sediment gets into the backfill filter, which requires a large volume of clean water to wash it.
- There may not be enough water passage time for chemical reactions between the coagulant and colloidal substances.
It makes sense to use pressure coagulation only with a small organic content in the source water.
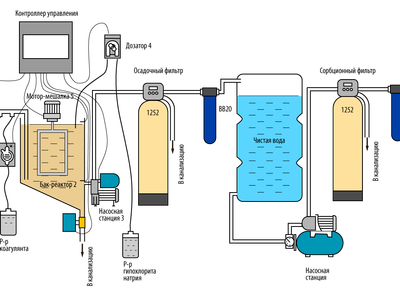
The figure below shows a scheme for removing organo-iron, iron and manganese by a non-pressure method. The source of such water can be a well, a river, a lake. The installation makes it possible to purify water with a content of increased permanganate oxidizability (1PO) of 40 units or higher. Water may contain 15 mg/I of iron, 1.5 mg/I or more of manganese. Also, the installation perfectly cleans turbid water with an odor and high color to the required sanitary standards.In addition, the equipment disinfects water.
Water purification by non-pressure coagulation
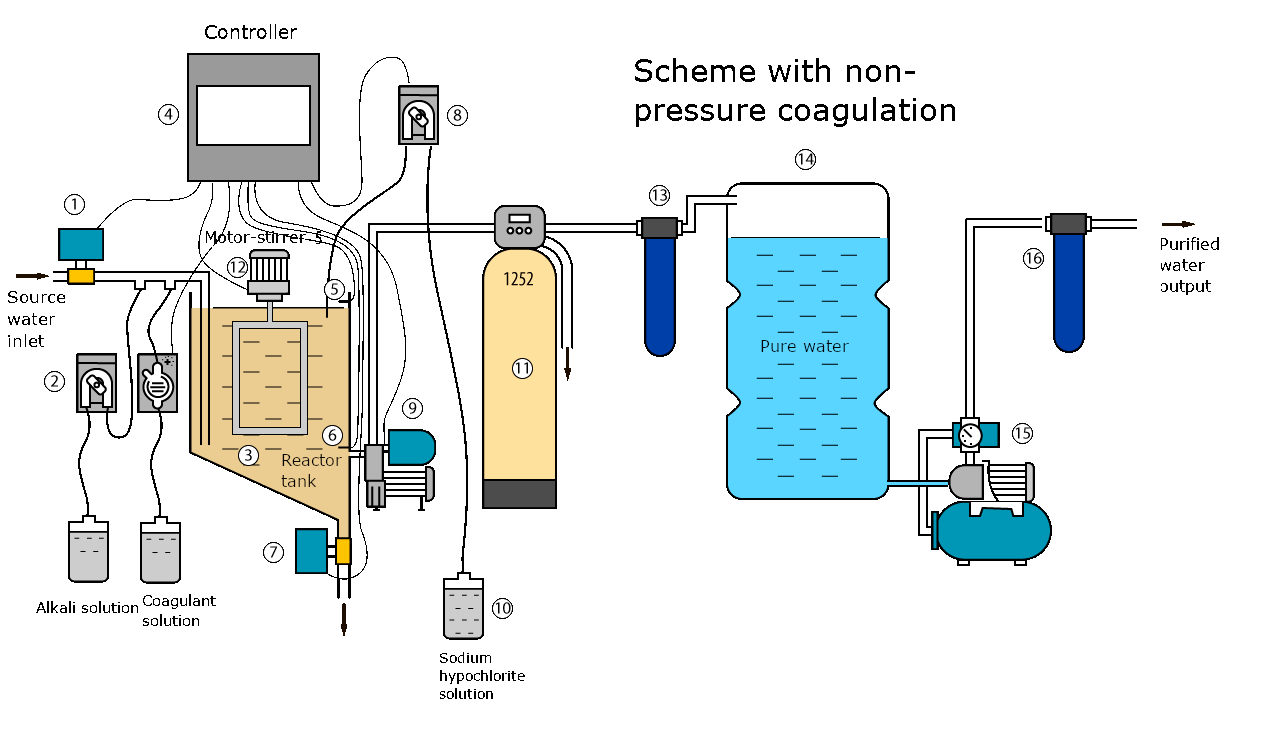
Description of the installation operation
At the command of the controller, an electric ball valve (1) opens and the reactor tank (3) is filled with source water. At the same time, continuous dosing pumps (2) mix a working solution of coagulant and an alkali solution into the source water. After filling the reactor with water, the controller (4) closes the ball valve (1) at the signal of the level sensor (5).
Then the colloidal substances in the source water react with the coagulant and fall to the bottom of the reactor tank in the form of flakes (3). This process can take several hours. The dose of coagulant and the sediment settling time are selected experimentally for a specific water.
At the end of filling the reactor tank, the agitator motor (12) is switched on for a while to accelerate the coagulation reaction and the formation of sediment flakes.
60-90 minutes after filling the reactor tank, when most of the sediment has fallen to the bottom, the metering pump (8) turns on for a certain time and dispenses a working solution of sodium hypochlorite into water. At the same time, the second mixing of water in the reactor takes place. Sodium hypochlorite oxidizes ferrous iron and manganese, and also disinfects water.
After 2-4 hours, at the end of settling, the controller (4) turns on the pumping station (9) and the clarified water is pumped into the pure water storage tank (14) through sedimentary (11) and cartridge (13) filters.
At the end of the pumping cycle from the reactor to the clean water tank, the controller (4) switches off the pumping station (9) at the signal of the level sensor (6) and opens the electric ball valve (7). The remaining water with sediment at the bottom is drained into the sewer from the reactor tank.
Then the cycle repeats:
1. Filling of the reactor tank with dosing of coagulant and alkali.
2. Dosing of sodium hypochlorite.
3. Sediment settling.
4. Pumping and filtration of clarified water.
5. Draining water with sediment.
From the storage tank (14), water is supplied to consumers by a pumping station (15) through a filter with a carbon cartridge (16). Activated carbon is necessary to remove chlorine and its compounds from water.
Advantages of this non-pressure coagulation scheme:
- It effectively purifies water with a Significant excess of organic matter, iron and manganese (including high-color peat and swamp waters).
- A significant part of the sediment (coagulated organic matter) simply drains into the sewer and does not enter the filters.
- Full automation of technological processes of water purification is achieved through the use of programmable relay control in the controller. During operation, the programmable relay is easily reconfigured when the quality of the source water changes.
An example of the configuration of such a water treatment plant